Learn how to tell if someone has COVID-19 or the flu here.
COVID-19 and the flu can cause similar symptoms. However, there are several differences between them.
The novel strain of coronavirus (SARS-CoV-2) causes coronavirus disease 19 (COVID-19).
Both COVID-19 and the flu are respiratory illnesses that spread from person to person. This article will discuss the differences between COVID-19 and the flu.
Symptoms
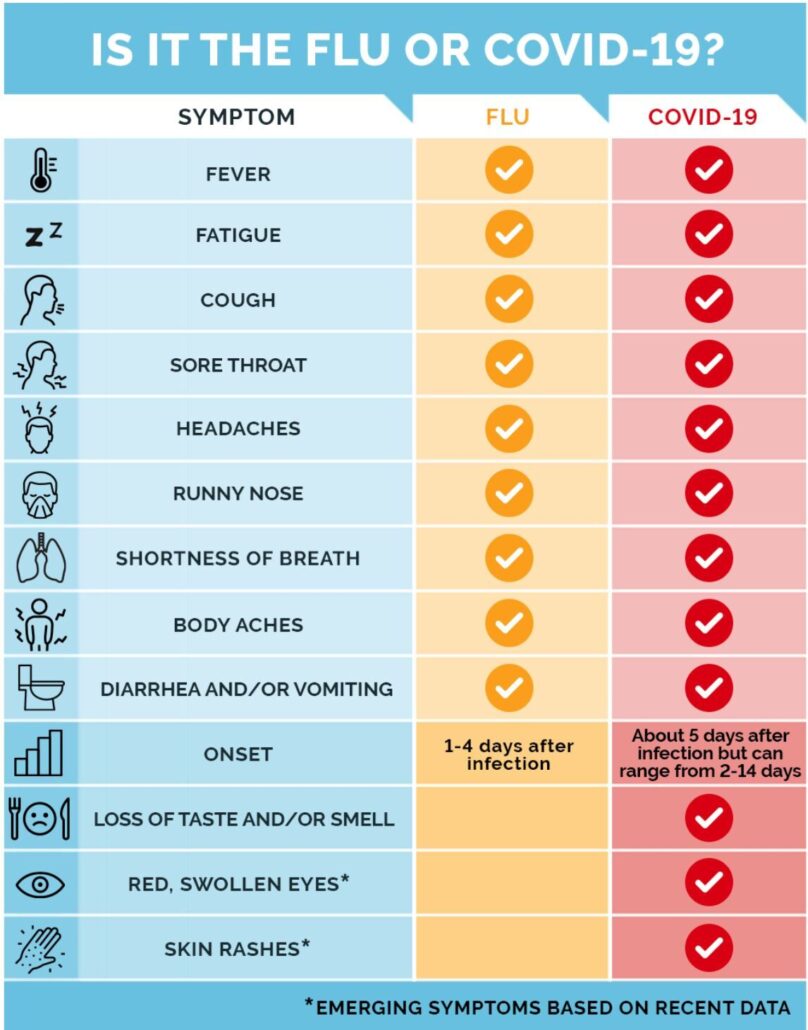
The symptoms of the flu and COVID-19 have some differences.
People who have the flu will typically experience symptoms within 1–4 days. The symptoms for COVID-19 can develop between 1–14 days. However, according to 2020 research, the median incubation period for COVID-19 is 5.1 days.
As a point of comparison, the incubation period for a cold is 1–3 days.
The symptoms of COVID-19 are similar in both children and adults. However, according to the Centers for Disease Control and Prevention (CDC), children typically present with fever and mild, cold-like symptoms, such as a runny nose and a cough.
The following table outlines the symptoms of COVID-19, the flu, and a cold.
Severity and mortality
The symptoms of COVID-19 and flu can range from mild to severe. Both can also cause pneumonia.
It is important to note that the World Health Organization (WHO) have classified mild symptoms of COVID-19 to mean that a person will not require hospitalization. The WHO classify mild cases to consist of symptoms including:
- fever
- cough
- fatigue
- loss of appetite
- sore throat
- headache
The CDC also lists the following as potential symptoms:
- breathlessness
- muscle pain
- chills
- new loss of taste or smell
According to the WHO, around 15% of COVID-19 cases are severe, and 5% are critical. Those in a critical state require a ventilator to breathe. The chance of severe and critical infection is higher with COVID-19 than the flu.
COVID-19 is also more deadly. According to the WHO, the mortality rate for COVID-19 appears to be higher than that of the flu.
Compared with the flu, research on COVID-19 is still in its early stages. These estimates may change over time.
Transmission
Both SARS-CoV-2 and the flu virus can spread through person to person contact.
Tiny droplets containing the viruses can pass from someone with the infection to someone else, typically through the nose and mouth through coughing and sneezing.
The virus can also live on surfaces. The WHO is not sure exactly how long the virus can survive, but it could be days.
According to the CDC, people can transmit the flu virus to people who are 6 feet (ft) away. According to the WHO, people should stay at least 6 ft away from anyone coughing or sneezing to help prevent the transmission of the SARS-CoV-2 infection.
According to the WHO, the speed of transmission differs between the two viruses. The symptoms of flu appear sooner, and it can spread faster than the SARS-CoV-2 virus.
The organization also indicate that people with flu can pass the virus on before they show any symptoms. A person can also pass on the SARS-CoV-2 infection even if they have no symptoms.
There are also differences in transmission between children and adults.
According to the WHO, the transmission of the flu from children to adults is common. However, based on early data it appears that it is more common for adults to pass the SARS-CoV-2 infection onto children. Children are less likely to develop symptoms.
The CDC recommend that people wear cloth face masks in public places where it is difficult to maintain physical distancing. This will help slow the spread of the virus from people who do not know that they have contracted it, including those who are asymptomatic. People should wear cloth face masks while continuing to practice physical distancing.
Treatment
As flu has been around much longer than COVID-19, there are more treatment options.
Most people with the flu do not require medical treatment. But a doctor might prescribe antiviral drugs in some cases, which can reduce the symptoms by 1–2 days.
These antiviral drugs help the body fight the virus. They treat symptoms and reduce how long the illness lasts.
There are currently no antiviral drugs approved to treat COVID-19, although scientists are currently researching drugs in trials. When scientists have had more time to study the disease, the availability of antivirals to treat COVID-19 will likely increase.
Although there is currently no approved treatment or vaccination for COVID-19, there are ways to help treat the symptoms and any complications that can occur.
For mild cases, a person should remain home and undertake social distancing. Healthcare professionals may prescribe antipyretics to reduce the fever.
For more severe cases, a person may require supplemental oxygen or mechanical ventilation on a breathing machine to treat the respiratory problems that may occur.
Prevention
The most effective way of preventing the flu is through vaccination.
Many strains of influenza can cause infection. The most common strains vary depending on the season.
Doctors will try to predict what strains will be most common each season to select the right vaccine components.
The best way to prevent spreading the SARS-CoV-2 virus includes:
- washing hands regularly
- avoiding touching the face
- keeping at least 6 ft away from anyone sneezing and coughing
- covering the mouth when sneezing or coughing
- staying at home if feeling unwell
- working from home if possible
- avoiding crowds and gatherings of any size
Causes
Both COVID-19 and the flu are viral infections.
Viruses are tiny microbes that survive by invading other living cells. These cells become host cells to the virus, which multiplies inside of them. They can then spread to new cells around the body.
Coronaviruses are a family of viruses that cause respiratory infections. The SARS-CoV-2 causes the infection that leads to COVID-19.
There are two types of viruses that cause the flu — influenza A and B. There are also several subtypes of influenza A. Any of these viruses can cause the flu.
Summary
COVID-19 and flu share some similar symptoms. The symptoms of flu tend to occur faster and can have greater variation. But COVID-19 is more likely to lead to severe illness or death.
Both viruses spread via person to person contact. Flu spreads faster and is more likely to affect children.
As the flu has been around longer, there are several effective antiviral treatments and vaccines available. Researchers and scientists are developing these for COVID-19, but treatments and vaccines are not likely to be available soon.
The best way to prevent COVID-19 is to practice social distancing, which means avoiding any non-essential social contact or travel. It is essential to maintain good personal and domestic hygiene by washing the hands regularly and keeping surfaces and utensils clean.
Viral infections cause both COVID-19 and the flu. But COVID-19 is due to the SARS-CoV-2 virus, and flu is from influenza A and B viruses.